by Barbara Nevins Taylor
I received the Moderna COVID booster shot at a CVS Pharmacy on Friday afternoon, September 3, 2021. Great. I was a little dizzy but I waited a bit and felt fine.
I rode my bike to the gym and I was elated. I felt protected from the delta variant.
But then at 8 p.m., we watched CNN and heard that the FDA wasn’t ready to approve the Moderna booster because Moderna had not provided enough data. My high shriveled away to nothing.
My husband Nick Taylor got his Pfizer booster the same day at another CVS Pharmacy and the news for him was better. The Pfizer dose was likely to win FDA approval very soon.
So what did getting the Moderna booster six months after I got my second shot mean? Was there something wrong with Moderna and would I and the others who got the booster get sick? Why didn’t the company provide the data in a timely way? And why had the CDC said, in August, that it was giving conditional approval for some people for the Moderna booster? The CDC website said, “CDC recommends the additional dose of an mRNA COVID-19 vaccine be administered at least four weeks after a second dose of Pfizer-BioNTech COVID-19 vaccine or Moderna COVID-19 vaccine.”
In other words, the CDC had recommended booster shots that the FDA had not yet approved. The incessant talk about the booster was incredibly confusing. I’m an information provider, and even so I wished the people talking and writing about the COVID booster would shut up unless they could clearly explain why the FDA was holding up approval. Everything they said added another layer of confusion.
I should say here, that because of my exposure to the air on 9/11 my lungs probably can not withstand a COVID attack of any kind. I had two small cancerous tumors removed from my left lung and my right lung has flecks of what doctors call “ground glass.”
That’s why I listened-up when the Department of Health and Human Services (HHS), on August 18, said in a news release, “Based on our latest assessment, the current protection against severe disease, hospitalization, and death could diminish in the months ahead, especially among those who are at higher risk or were vaccinated during the earlier phases of the vaccination rollout. For that reason, we conclude that a booster shot will be needed to maximize vaccine-induced protection and prolong its durability.”
There was no news about the Johnson & Johnson vaccine because that came months after the first vaccines were given. We’re waiting.
The White House said it would begin the booster roll-out on September 20th. They wanted to coordinate it to avoid the chaos that resulted with the first vaccines because the Trump administration had no plan.
Nearly 1 million people didn’t wait for September 20. We forged ahead and got the vaccine because we had hit the five and six month mark after our second shots. Israeli scientists suggest that five months protection against the delta variant was the most to expect from the Pfizer vaccine.
This time it was easy. The COVID booster was available at our local pharmacies and we made appointments.
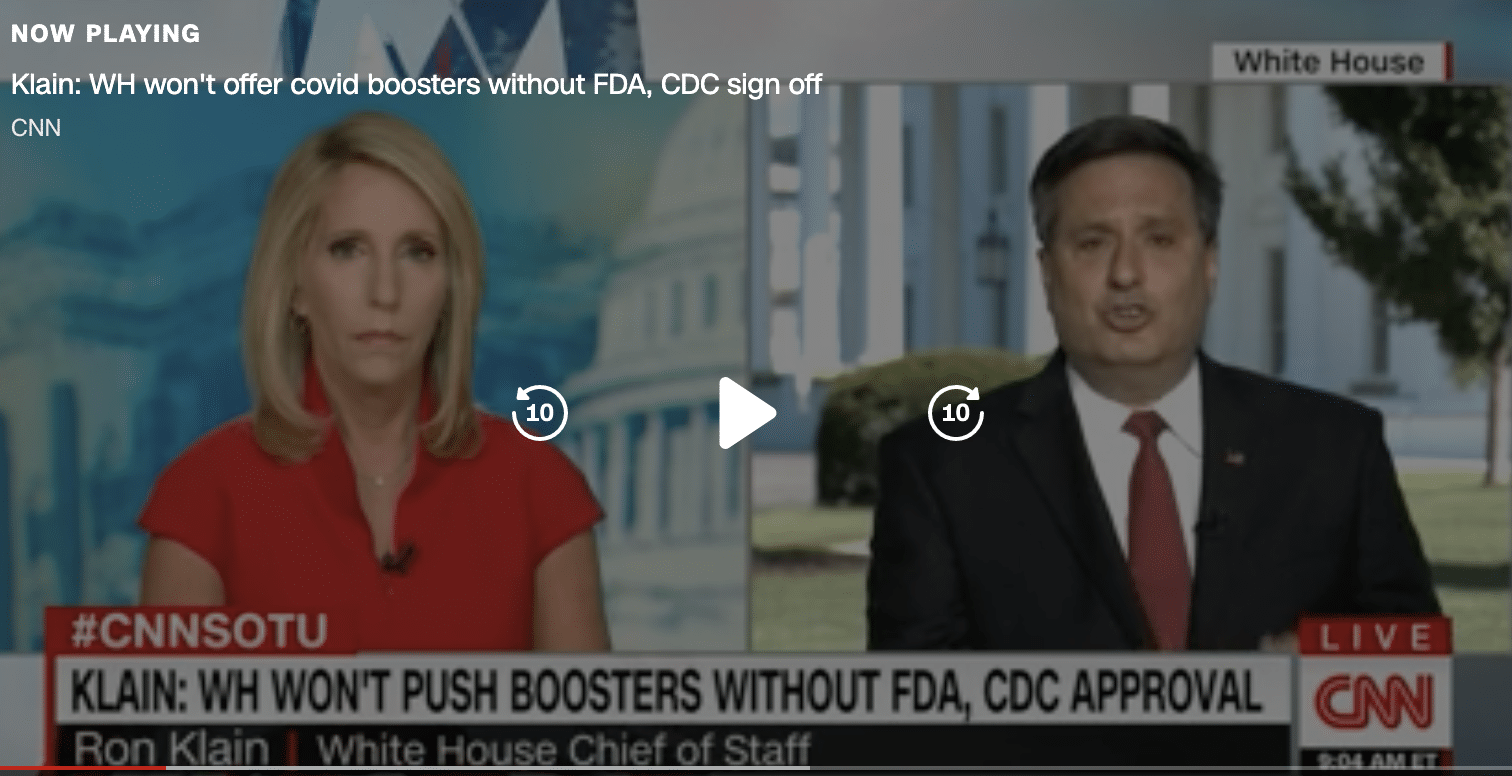
But on Sunday September 5 White House Chief of Staff Ron Klain told Dana Bash on CNN, “No one is going to get boosters until the FDA says they are approved, until a CDC advisory committee makes a recommendation.”
Come on. It was like a throwback to the Trump era of lies and double-talk. We thought that was over. Again, nearly 1 million, according to the CDC, received the booster.
On the same Sunday morning, the straightest COVID talker, Dr. Anthony Fauci, weighed in on CBS’s Face The Nation.
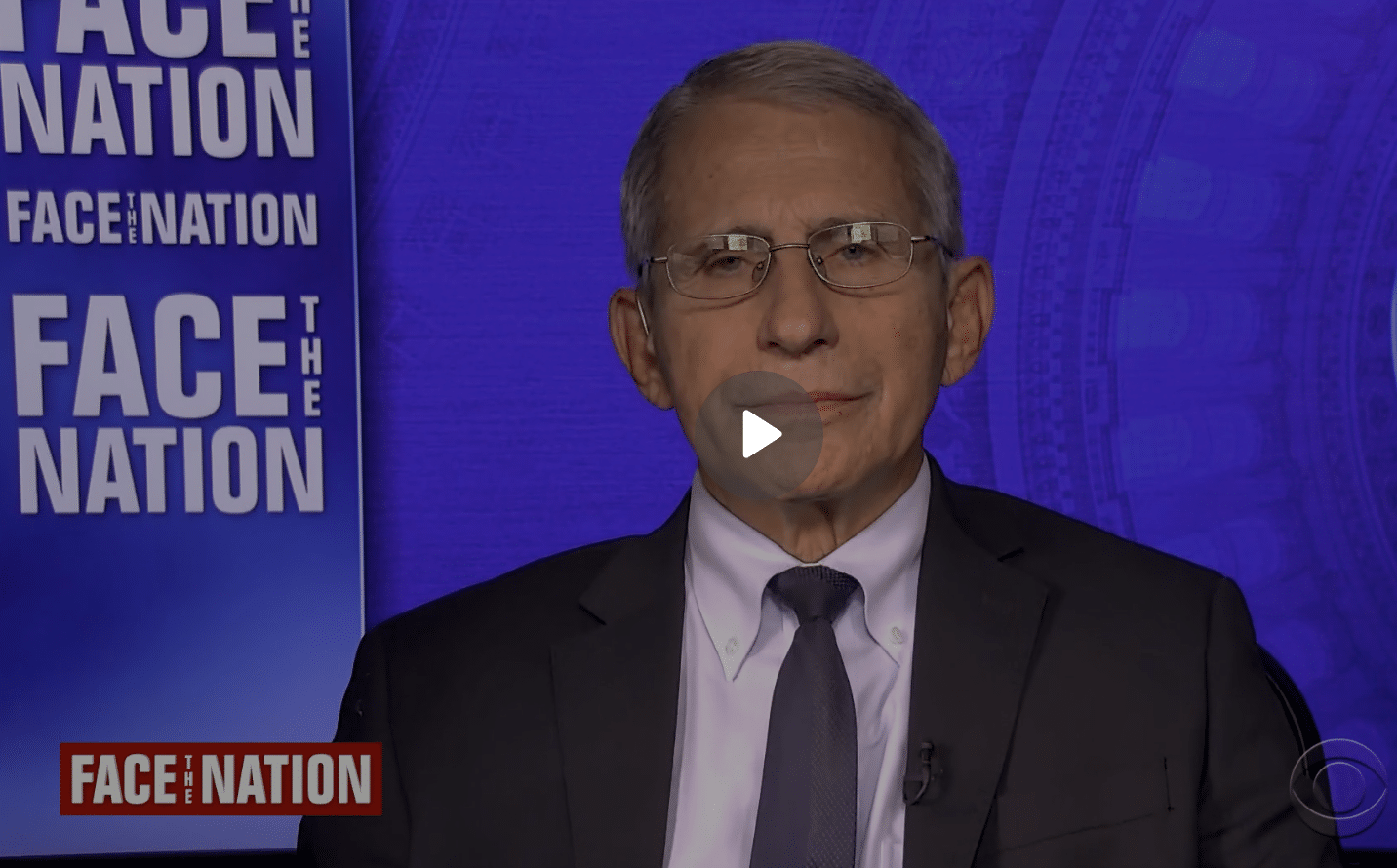
He told Weija Jang that he expected the Pfizer booster would be approved by September 20, and Moderna would follow. He said, “Looks like Pfizer has their data in, likely would meet the deadline. We hope that Moderna would also be able to do it so we could do it simultaneously, but if not, we’ll do it sequentially. So the bottom line is very likely, at least part of the plan will be implemented, but ultimately the entire plan will be. “
We appreciate the fact they want to follow the science and are waiting for the FDA to review the data from Pfizer and Moderna. But the vaccine is available and they pointed out that it was okay for people who were immune-compromised to get the vaccine before the big rollout.
Now there is a lot of vaccine out there and a lot is going to waste. NBC News reported that states have thrown away 15.1 million doses since March. People who are eligible should get the first, second and third shots, if they are safe. We want to end this COVID nightmare and it’s clear from the data that if we are unvaccinated more people will get sick and more people will die. 153, 246 people contracted COVID during the past seven days and that’s up 4.9 percent from the same time last year, according to the CDC. The New York Times reports that U.S. is averaging more than 1500 deaths a day for the first time since March.
In the meantime, Moderna needs to provide the data that is acceptable to the FDA about the booster. Those of us who received the third dose, and those who need it should know where we stand. In a news release on September 1 Stéphane Bancel, Chief Executive Officer of Moderna, said, “We will continue to generate data and transparently share to support governments and regulators as they make evidence-based decisions regarding future vaccination strategies.”
Please hurry up.
Oh, and side-effects from the Modern booster: I was very achy and tired the next day. By the middle of the second day, I felt like myself.




